Miyoshi Lab
We investigate how genetic defects affecting active molecular transport cause hereditary hearing loss in humans.
Overview
The inner ear is a biological mechanosensor specialized for detecting sound and balance. In vertebrates including humans, these mechanical stimuli are sensed by the inner ear sensory hair cells equipped with brush-like, actin-based protrusions called stereocilia. Development of functional stereocilia depends on specific motor proteins—namely, myosins—that actively transport their “cargo” along the actin track in stereocilia, including components of the mechanoelectrical transduction (MET) machinery.
Our laboratory investigates how these myosin molecules localize themselves and their cargo within stereocilia. To address this question, we recently developed the STELLA (Single-Molecule Tracking and Enhanced Localization in Live Aural specimens) workflow, supported by NIH funding (1R00DC019949). This approach enabled us to demonstrate that MYO7A, a component of the MET machinery, moves processively—presumably as a dimer—toward stereocilia tips when released from its autoinhibitory conformation.
We are now employing STELLA to investigate how the motor activities of MYO7A and other myosins contribute to stereocilia development. By experimentally linking genetic defects in active cargo transport to both morphological and functional abnormalities in stereocilia, we aim to define the pathogenic roles of myosin and cargo variants found in patients with hearing loss and ultimately to develop tailored therapeutic strategies, including gene therapy and pharmacological rescue.
We are pleased to share that our latest article has been published in Nature Communications on September 1, 2025:
Single-molecule fluorescence microscopy reveals regulatory mechanisms of MYO7A-driven cargo transport in stereocilia of live inner ear hair cells
A short article summarizing the key points is also available on the Behind the Paper blog by Springer Nature:
Myosin 7A is a Miniature Molecular Motor with a Major Mission
Takushi Miyoshi, MD, PhD Primary Investigator
Current projects

MYO7A and assembly of the MET machinery

Trafficking of MYO15A, the stereocilia elongator
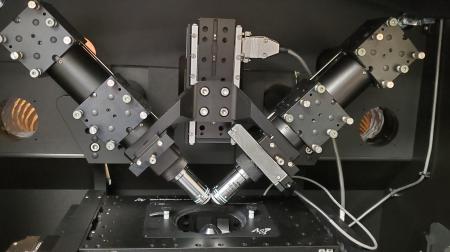
Technical advances in single-molecule microscopy in live hair cells
Research focus
The central goal of our lab is to elucidate how the motor activity of MYO7A is regulated in stereocilia. We recently demonstrated that MYO7A likely traffics as a dimer, consistent with previous studies on cargo-mediated dimerization of this myosin. However, it remains unknown what factors release MYO7A from its autoinhibitory conformation and trigger its dimerization (or possibly multimerization) in hair cells. We are currently investigating physiological activators of MYO7A trafficking, which may serve as key molecules for regenerating stereocilia with functional MET machinery.
We are also expanding our imaging efforts to include MYO15A, the stereocilia elongator. This myosin resembles MYO7A in its motor domain, whose kinetic properties are compatible with processive movement as a dimer along actin filaments, as well as in its overall domain architecture. However, MYO7A and MYO15A interact with distinct cargo proteins and play different roles in stereocilia development. We are examining how MYO15A reaches stereocilia tips and promotes actin filament elongation.
Finally, we are advancing our STELLA workflow to achieve higher spatial resolution and sensitivity by leveraging cutting-edge microscopy platforms. Tracking of myosin molecules is also being enhanced through artificial intelligence (AI)-based methodologies, in collaboration with the SIU School of Computing and Engineering. These advances will enable more detailed analyses of myosin dynamics in stereocilia and provide a technical foundation for detecting aberrant kinetics of myosin and cargo variants found in patients with hearing loss.
Publications
Alumni
Mrudhula Sajeevadathan, PhD
Join our lab
Graduate students (Master's or PhD): Department of Biomedical Sciences
Our lab currently has openings for graduate students at the Master’s or PhD level. We welcome applicants interested in elucidating the pathological mechanisms underlying human hearing loss or in developing single-molecule biochemical assays using live cells or solutions. We are particularly looking for students who are eager to grow as independent scientists through hands-on research that integrates molecular and cell biology, fluorescence microscopy, human genetics, and image analysis.
For more information, contact Takushi Miyoshi.