Welcome from Andrzej Bartke, Director of Geriatric Medicine and Professor of Physiology and Medicine
 We are working on the genetic control of aging in mammals and on endocrine mechanisms responsible for the effects of longevity genes. We reported the first evidence that mutation of a single gene can significantly extend life span in a mammal (Brown-Borg et al. Nature 384:33, 1996). We have extensively characterized the phenotype of long-lived Ames dwarf (Prop1df) mice and identified several mechanisms that are likely to explain or contribute to delayed aging and greatly prolonged longevity of these animals. These mechanisms include reduced plasma levels of insulin-like growth factor 1 (IGF-1), reduced growth and adult body size, reduced incidence of cancers and delayed development of fatal neoplastic disease, reduced plasma insulin and glucose levels, improved sensitivity to insulin, increased levels and/or activity of several antioxidant enzymes, and reduced oxidative damage of mitochondrial DNA. We have also shown that long-lived Ames dwarf mice maintain youthful levels of cognitive function (learning and memory) into advanced age.
We are working on the genetic control of aging in mammals and on endocrine mechanisms responsible for the effects of longevity genes. We reported the first evidence that mutation of a single gene can significantly extend life span in a mammal (Brown-Borg et al. Nature 384:33, 1996). We have extensively characterized the phenotype of long-lived Ames dwarf (Prop1df) mice and identified several mechanisms that are likely to explain or contribute to delayed aging and greatly prolonged longevity of these animals. These mechanisms include reduced plasma levels of insulin-like growth factor 1 (IGF-1), reduced growth and adult body size, reduced incidence of cancers and delayed development of fatal neoplastic disease, reduced plasma insulin and glucose levels, improved sensitivity to insulin, increased levels and/or activity of several antioxidant enzymes, and reduced oxidative damage of mitochondrial DNA. We have also shown that long-lived Ames dwarf mice maintain youthful levels of cognitive function (learning and memory) into advanced age.
We have performed similar studies in long-lived growth hormone receptor knockout (“Laron dwarf”) mice produced by Zhou et al. in the laboratory for Dr. John Kopchick and demonstrated numerous similarities in the characteristics related to aging and longevity in Prop1df and GHR-KO mice. These findings, along with results from other laboratories firmly establish physiological role of the somatotropic axis (GH and IGF-1) in the control of aging in mammals.
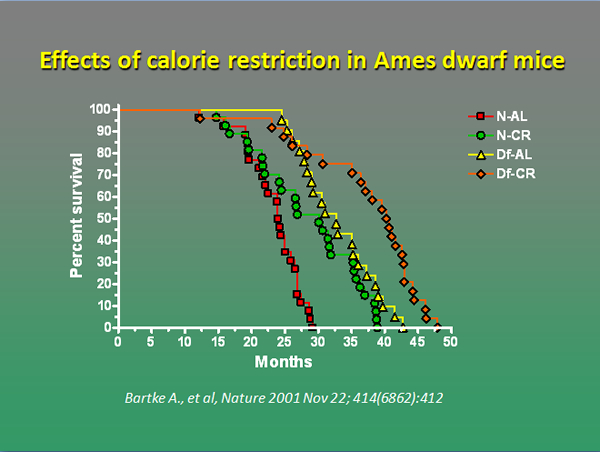
We are currently examining interactions of mouse longevity genes with caloric restriction and we have already demonstrated that 30% reduction of food intake leads to further, significant extension of the average and the maximal lifespan in Ames dwarf mice (Bartke et al., Nature 414:412, 2001). Studies of expression of genes related to GH, IGF-1, and insulin signaling and analysis of broad profiles of gene expression suggest that Prop1df and GHR-KO prolong life by mechanisms that are overlapping but definitely not identical to the effects of caloric restriction.
We are currently using Ames dwarf and GHR-KO mice to identify candidate genes for studies of gene polymorphisms related to longevity in the human. This involves close collaboration with a number of laboratories involved in the study of human aging. We are also beginning to examine effects of hormone replacement therapy during development of long-lived mutant mice on insulin signaling and longevity. Additional projects involve studies of transgenic mice overexpressing growth hormone, which are insulin resistant and short lived, and a study of the interactive effects of longevity genes, GH overexpression, and caloric restriction on reproductive development and fertility.
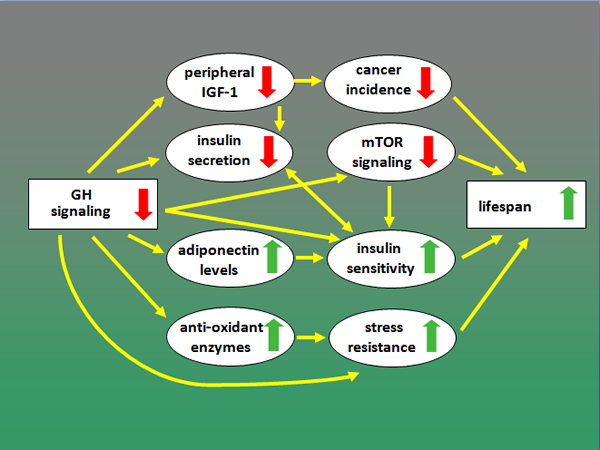
Our current ideas concerning mechanisms that link reduced activity of the somatotropic axis with prolonged longevity are summarized in a diagram below.