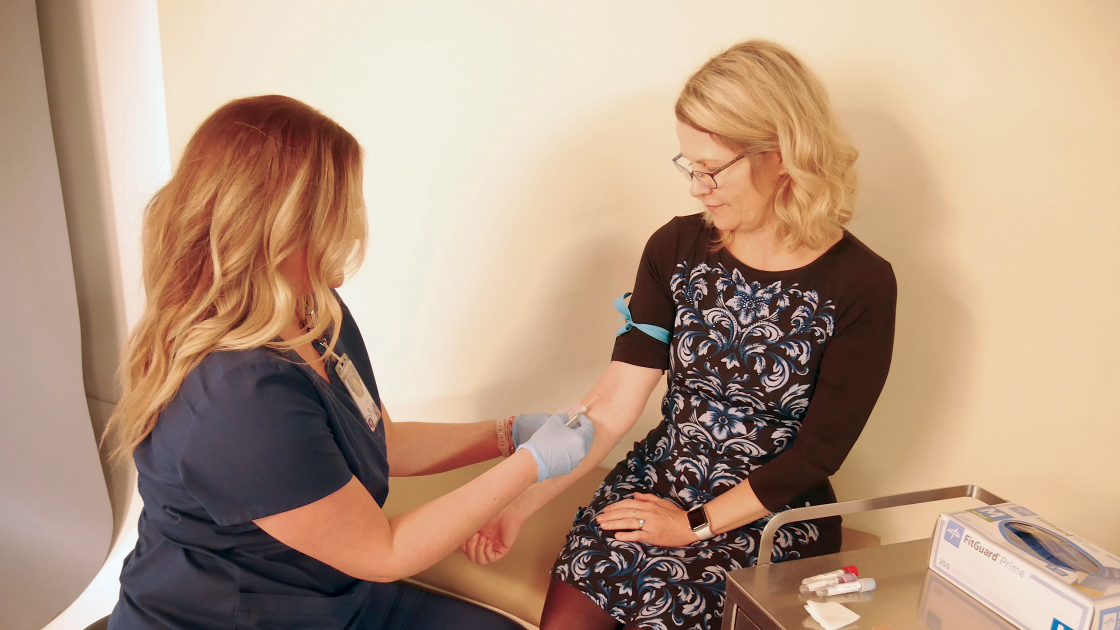
Operations Division
The Operations Division assists in planning, initiating, and conducting clinical trials. The division works closely with sponsors, investigators, and the IRB to facilitate the successful conduct of clinical trials that meet the regulatory requirements that govern human research.
The Operations Division will:
• Facilitate, foster, cutting-edge, and support the conduct of high-quality clinical research
• Increase studies that provide patients with access to, research opportunities
• Serve as a shared resource to investigators and research coordinators.
Services include:
• Research nurse/coordinator support for study start-up, follow-up, and closeout
• Screen and identify eligible patients
• Contract and budgeting
• IRB preparation, submission, and continuing review
• Educational opportunities

National recognition. Regional impact.
“This recognition confirms what we at SIU have always known: that world-class research is happening right here in central and southern Illinois.” – Dr. Jerry Kruse
